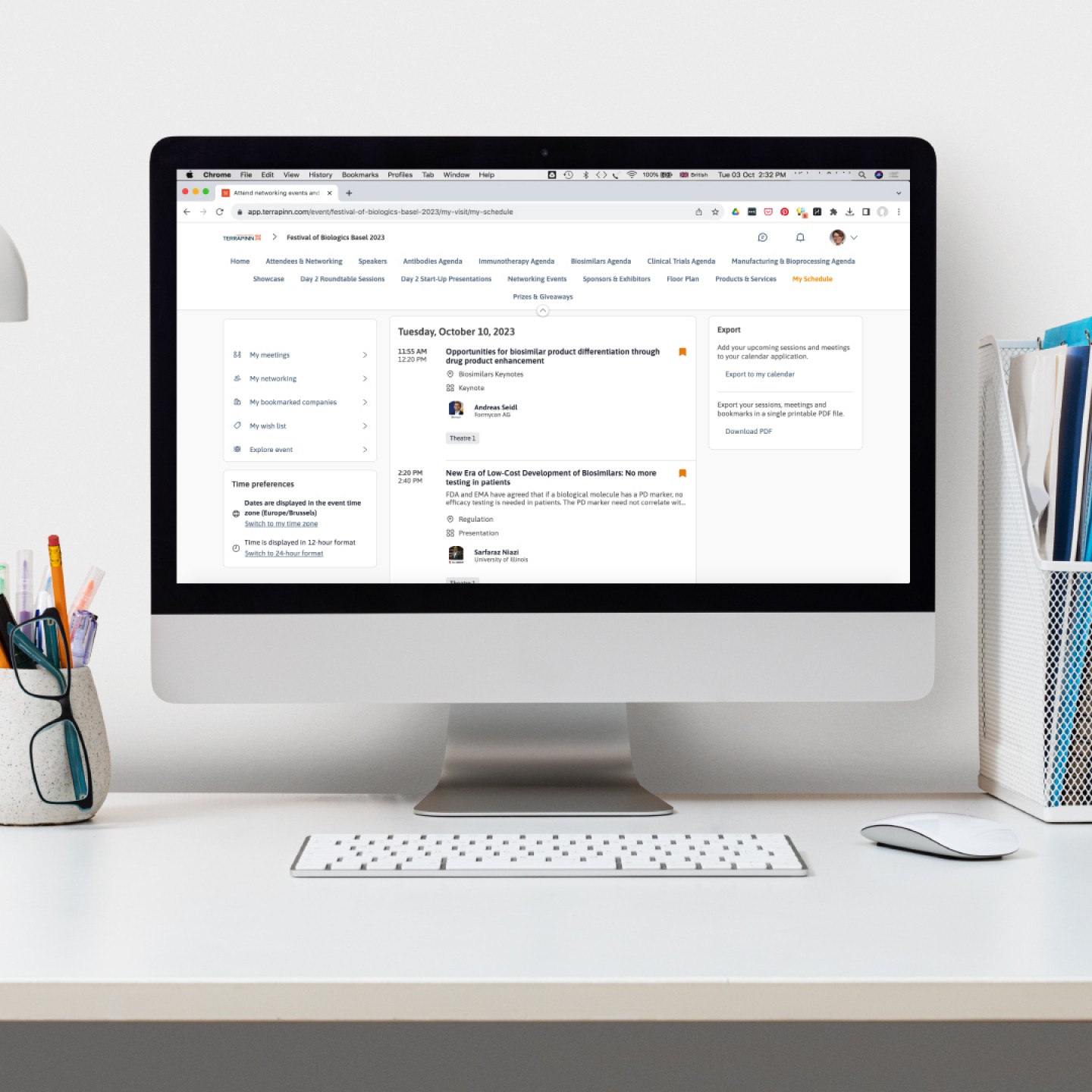
With the thoughtfully curated program and the practical events app, our Biologics schedule is quickly filling up.
So far we have enrolled for the following keynotes:
• Opportunities for biosimilar product differentiation through drug product enhancement, presented by Andreas Seidl from Formycon AG
• New Era of Low-Cost Development of Biosimilars: No more testing in patients, presented by Sarfaraz K. Niazi from the University of Illinois Chicago
• ATL-105, a new broadband antiviral therapy for inhalation, presented by Dr. Rüdiger Jankowsky from AATec Medical GmbH
• JJP-1008: A Novel Checkpoint Inhibitor for Reversing Immunosuppressive TME in Cancer Treatment, presented by Louis Boon from JJP Biologics
• Engaging the Full Immunity Cycle with Innate Cell Engagers (ICE®): New Therapeutic Avenues for Patients, presented by Anna Lisa Guendner from Affimed GmbH
Between the keynotes, there are still some open slots for meetings. Please contact us if you would like to discuss QP Certification, GMP, or Import to the EU.